To Be Seen as Generated and Fashioned by Volition
Introduction
Libet et al. (1983) used a fast-rotating clock to demonstrate in salubrious subjects that the perception of having willed voluntary movement was preceded past cortical activity measurable by electroencephalography (EEG). Their subjects identified on the clock at what time they decided to move (time W), and when they had the sense that they moved (fourth dimension M). The EEG measure was the Bereitschaftspotential (BP), a long, slow EEG negativity over the vertex that begins about 1 south before movement. They concluded from these results that the sense of deciding to act may not actually represent a conscious conclusion, but merely be a conscious awareness arising in the eye of an unconscious process that precedes movement.
Since the Libet et al. (1983) experiment, many criticisms have been raised against his timeline of voluntary motion. For example, Haggard et al. (1999) proposed the problem of "prior entry bias," in which events that crave more than attention are perceived equally happening earlier. Gomes (2002) constitute error with the requirement to delineate Due west and Chiliad – phenomena which he finds to be duplicate as most people have a "unitary sensation" of voluntarily moving.
These objections highlight the demand for further studies into the questions raised by Libet et al. (1983) initial experiment. Many movements, such every bit walking, are executed and even corrected without sensation. The driving interest backside this research is and then not but why the mind should exist "behind" the brain in awareness of move, merely why awareness is necessary at all. Schizophrenia represents a unique instance against "unitary sensation," every bit many patients with schizophrenia have one component of this awareness (the sensation of move) without the other (the awareness of deciding to move, or at the to the lowest degree, misattributing the source agent of Due west) (Graham et al., 2014).
Schizophrenia comprises a heterogeneous grouping of mental disorders characterized past disturbances in class and content of thought, altered mood, and impaired perspective of self and external environment and includes symptoms like passivity (Herbener and Harrow, 2019). Passivity symptoms demonstrate a crucial, subjective change in how self is experienced and allows for an external amanuensis to substitute for the cocky in generating thoughts, sounds, and movements (Gallagher, 2004; Graham-Schmidt et al., 2018). Passivity phenomena can then act as a model for agreement how the normal conjoining of a motor activeness with the intention to move tin can unravel and leave a person susceptible to delusions of external control. Moreover, passivity was found related to the dysfunction of visuomotor activeness monitoring, suggesting that psychotic passivity experiences might event from abnormal fundamental activeness monitoring mechanisms (Schnell et al., 2008). Another possible role would be played by the corollary discharge (CD), a feedforward machinery that normally contributes to the emergence of the sense of agency, and that appears to be altered in schizophrenia (Poletti et al., 2019). A study found that the microstructural integrity in the pathway connecting frontal heart fields (FEFs) with the mediodorsal thalamus (Dr.) was compromised in patients affected by schizophrenia. This was seen related to an oculomotor CD dysfunction and the severity of psychotic symptoms (Yao et al., 2019).
Healthy people, such as in Libet et al. (1983) study, are able to identify an experience of will that precedes voluntary movement and know "I moved." With W before M, these people take an awareness of the intention to movement and tin can appropriately attribute causality (Wegner, 2003). The purpose of this study is to determine the timing of willing and initiating a move in a disease state where the awareness of intention and action are impaired. We hypothesize that in schizophrenia patients, the separation between W and M will be shorter than in healthy volunteers, resulting in an altered sense of causality and hence conscious will. Moreover, we hypothesize that this contradistinct perception of W is related to the presence and the severity of passivity phenomena.
Materials and Methods
Report Participants
Fifteen patients with DSM-IV diagnosis of schizophrenia (American Psychiatric and Clan, 1994) who had volunteered to participate in the clinical research unit of measurement of the Clinical Encephalon Disorders Branch at the National Establish of Mental Health (NIMH) were enrolled. One patient withdrew from the report due to alter in wellness status. The enrolled patients were 4 women and 10 men (hateful age 28.9 years, range from 19 to 57 years). Fifteen healthy, age-matched volunteers were recruited; i withdrew due to change in health status leaving six women and viii men (mean historic period 31.three years, range from 18 to 57 years). One field of study decided to withdraw after signing the consent but before participating in the study. All patients were under second-generation antipsychotic handling, as required by the National Institute of Neurological Disorders and Stroke Institutional Review Board who approved this written report. All patients were clinically stable at the time of testing. Patients were asked to requite informed consent. Subjects were able to demonstrate that they understood, amidst other factors, that participation was voluntary, that it would not benefit them, and that they could withdraw from the study at whatever time. Patients likewise demonstrated that they understood that not participating was an pick. Every bit part of documenting informed consent, patients were asked to have a written examination covering details of the protocol and its benefits and risks. Patients who were unable to score at to the lowest degree 75% on the written test were excluded.
A neurologist performed the history and physical exam for schizophrenic patients and healthy volunteers. Patients on the clinical inquiry unit had to come across rigorous criteria in order to participate in inquiry. Exclusionary criteria included history of traumatic encephalon injury, known comorbid neurological disorders, including epilepsy, history of drug and alcohol abuse. A psychiatrist administered clinical rating scales [i.due east., appropriate sections of the Structured Clinical Interview for DSM-IV (SCID)] (First et al., 2002). Moreover, since patients with schizophrenia often exhibit deficits in numerous neurocognitive domains, including attention, the psychiatrist administered also a comprehensive battery of neuropsychological tests to decide whether the patients were able to participate the written report. This battery included Wechsler Memory Scale-Revised (Wechsler, 1987), Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981), Trail Making Test A and B (Tombaugh, 2004), and the vigilance and distractibility versions of the continuous performance task (CPT) (Gordon and Mettelman, 1987).
Times S, M, W
All participants watched a training video on the tasks. The researchers followed a standardized script when giving task instructions. The good for you subjects and schizophrenic patients watched a clock presented on a computer screen. The clock was a circumvolve with a cherry dot, which revolved around the clockface in 3 s. A circular scale was marked effectually the periphery of the clock, with tick marks at each "5 south" position, actually marking 250 ms of real time (Figure 1). At that place were three sessions, South, Due west, and Grand. During the session S, subjects received a curt somatosensory stimulus; during the session W and M they had to motility spontaneously. The estimator with the clock recorded the time somatosensory stimuli were given, also as the electromyographic (EMG) onset of movement.
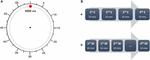
Effigy 1. Experimental job sequence. (A) During every task, the participants were asked to prepare the center of the clock (replica of clock used in the study). The fast-moving cerise dot rotated clockwise around the perimeter of the clock with a total revolution accomplished in 3 s. The time was reported every bit for a common clock, so every v south mark was actually 250 ms. (B) The participants were asked to study the time (S) of external electric stimulation (A) in a separate session, before performing the job with the W and M conditions. W and M were alternated in 4 blocks of 10 movements each. The participants were asked to report every time aloud, subsequently every trial.
Fourth dimension S was measured past sessions in which subjects watched the clock and noted the time at which they perceived a short somatosensory stimulus (time South), repeated 40 times. The stimulus was a non-painful electrical shock of an intensity set up at twice the personal sensory perception threshold. The magnitude of the non-painful stimulation was established by a series of increasing stimulus intensities to make up one's mind the subjective sensory threshold. This evaluation was performed during a separate session before the proper experiment. The stimulus consisted of a ii ms pulse, delivered by a ring electrode. In one of the analyses, the sensory stimulation trials were used to adjust the interpretation of times W and M. Moreover, it was a useful control for the power of the bailiwick to written report with a minimum accuracy the time of an objective movement. By Libet et al. (1983) procedure, we subtracted S from W and M before reporting these times as results.
Times Westward and M were measured in sessions in which subjects were asked to make a voluntary motility with the right arm approximately every five–6 turns of the clock. The subjects voluntarily made a brisk move of wrist and finger extension, which was relatively stereotyped, rapid and curt in duration. During this session subjects alternated betwixt blocks of noting the time at which they had the conscious intention to move, Westward (or felt that some external force ordered their move), and the fourth dimension at which they felt the initiation of movement M. The blocks consisted of 10 movements each, alternate between Westward and Chiliad as the reported time. Four blocks each of Westward and M were studied in total. Subjects were asked to annotation the time of Due west, K, and S and and so wait until a few rotations of the clock have passed earlier proverb the time aloud. This will forbid the presence of artifacts (due to head movements or speech) that would have affected the electrophysiological recordings. Furthermore, following Libet's procedure, every participant was instructed to keep their gaze on the middle of the clock, even when they had to note the position of the manus of the clock, to avoid center movements that could take produced ocular electric artifacts. Finally, again in accordance with Libet's original experiment, the subjects were free to blink when they had the urge to so, but were instructed, after a blink, to look for at least another revolution of the clock before moving.
Electroencephalography and Electromyography
EEG was used to record brain electrical potentials throughout the experiment. A standard EEG electrode cap, with 28 electrodes placed co-ordinate to the international 10–20 organization, was used and placed using standard measurements. Impedance was kept at less than v kOhm. The EEG was filtered with a DC-200 Hz low-pass filter and digitized at a rate of 1000 Hz. The EMG indicate was recorded from disposable surface silverish-silverish chloride electrodes over the right extensor digitorum communis in a bipolar montage. The EMG betoken was rectified, integrated, and fed to a homemade Schmidt trigger that was prepare to trigger at the EMG flare-up onset. An electro-oculogram (EOG) was recorded in all subjects. Each session was stored in digital format for off-line analysis.
EEG and EMG Analysis
For all the EEG and EMG analyses we followed the methods delineated past Karp et al. (1996). Recordings were visually inspected off-line. Artifacts and insufficiently brisk movements were excluded from analysis. The EOG recording was used to verify the ocular atmospheric condition in every trial, detecting the epochs afflicted by blinks, saccades, and generic eye movements. During W and Thousand trials, epochs were marked and segregated co-ordinate to whether the discipline was reporting West or M. Data were epoched appropriately to the rectified EMG burst onset, considered the trigger for the motion. Specifically, every epoch had a duration of four s, starting 3500 ms before trigger, until 4500 ms after the trigger. Since the premotor potential onset is defined by a rise in negativity above baseline (established every bit the mean amplitude from approximately −3500 to −3000 ms before EMG onset) in the averaged tracings, the amplitude of every potential was referred to that baseline activity. The voltage analysis was focused on the motility-related cortical potentials (MRCPs). In item, the MRCP on the vertex (electrode Cz) was divided into the classical two components: the Bereitschaftspotential 1 (BP1), the before widespread component, and the Bereitschaftspotential 2 (BP2), the later steeper component. This was done for both the W and G trials.
Positive Symptoms
The calibration for assessment of positive symptoms (SAPS) (Andreasen, 1984) was used to appraise the severity and presence of psychotic symptoms in 12 out of the fourteen patients. Two patients declined the assessment. The passivity symptoms were derived by the following items of the SAPS, belonging to the Delusions domain: 15, delusions of being controlled; 16, delusions of mind reading; 17, thought dissemination; xviii, thought insertion; and 19, idea withdrawal (Frith, 2005; Schnell et al., 2008). The level range was 0 (none) to v (severe). The presence was considered with a score higher than 0, while for the severity the sum of the five items was considered. As a control assay, we similarly computed the Hallucinations domain (items 1–7), and the whole Delusions domain (items 8–20).
Statistical Assay
Noteworthy, Benjamin Libet and colleagues did non provide any statistical analysis, and the entire work was purely descriptive. Among the most recent Libet's replications involving patients, we institute that Jungilligens et al. (2019) compared healthy volunteers with patients afflicted by epileptic seizures. They used a MANOVA with the gene grouping and the score of scales of dissociating experience and W–Thousand difference value as dependent variables. Baek et al. (2017) used Libet's prototype with the fMRI. They divided patients with functional neurological disorders in ii groups, with or without positive motor symptoms, compared in a simple ANOVA model with healthy volunteers. Edwards et al. (2011) for a Libet'south replication with healthy volunteers and patients with psychogenic tremor used a ii-way ANOVA with the status (M, W) and grouping (patients and controls) as master factors.
Regarding the concept of psychotic symptoms, Schnell et al. (2008) hypothesized a human relationship between passivity symptoms and the BOLD response within the monitoring network during an object recognition task. To test information technology, they used a uncomplicated regression assay only in the patient grouping. Graham-Schmidt et al. (2018) tested the feel of lost agency in patients with Schizophrenia, using the projected hand illusion (PHI) with active and passive movements. The analyses were performed with a linear mixed model with questionnaire responses every bit the dependent variable and presence of passivity symptoms (Controls, Current, By, or Never), movement status (Active or Passive), and delay condition (Synchronous or Asynchronous) equally the fixed furnishings. Yao et al. (2019), using the probabilistic tractography, analyzed the integrity of the pathway projecting from the superior colliculus to the FEFs, via the MD. This pathway conveys oculomotor CD associated with saccadic eye movements in non-human primates. They used the Spearman correlation between the measure of the integrity of the path and the psychotic symptoms. In line with the previous experiments, we decided to use a linear model and a simple regression for the symptoms and the reported times but for the patients. Moreover, we used the S condition to right the reported times, every bit follows.
Due west and Chiliad times were compared using a mixed model where the measures for Grand and W (Status) represented the repeated factor and schizophrenia patients versus good for you volunteers were the grouping (Group) factor. The subjects were used every bit the random gene. Nosotros also included an evaluation of the Bayes factor (BF), as a complementary indicator to the classical hypothesis testing. This analysis was performed adapted for S. Mail service hoc testing was performed with pairwise comparisons of estimated marginal means (EMMs) with Tukey aligning. p-Values less than 0.05 were considered meaning. The same model was used to clarify the differences in the onset of the MRCPs (i.e., BP1 and BP2) and the amplitudes at the time 0 (onset of EMG). Depending on the normality of information, we performed Pearson'due south or Spearman's rank correlation to wait at relationship between passivity and psychotic symptoms in schizophrenia.
For all the statistical analysis, nosotros used R (version 4.0.ii, R Core Squad, 2018) and GraphPad Prism (version eight.4.0 GraphPad Software, LLC). The normality of the variable was assessed using the Shapiro–Wilk test. ROUT examination (Q = 1%, Motulsky and Dark-brown, 2006) was used to reveal the presence of eventual outliers.
Results
Differences in Times G and West
Reported times were normally distributed. For the S session (normality p = 0.v for the patients, p = 0.one for the volunteers) patients with schizophrenia reported feeling the sensory stimuli at 59.4 ms (SEM 12 ms) afterward the stimuli were practical. Healthy volunteers reported time S at 11.7 ms (SEM xiv.two ms) after stimuli onset. This difference was significant (p = 0.02). Time S was then used to adjust time W and M on an individual ground as discussed in the Methods. In Table one the three values were reported as hateful and standard error of the hateful for both the groups (see the Supplementary File for the individual values). ROUT exam revealed the presence of one outlier, which was removed from the statistical model. The mixed model with the reported values of M and W (adjusted for S) was pregnant for the Study type factor (df = 24, F = 7.6, p = 0.011, BF = ii.nine) and for the interaction Report type x Group (df = 24, F = v.nine, p = 0.02, BF = 2.nine). The BF related to the non-significant factors were smaller than 1. Postal service hoc comparisons showed that patients with schizophrenia did not report a significant divergence in the timing of Grand and Due west (xi.vii ms) (SEM 32.9, p = 0.98, event size = 0.12). Healthy volunteers past dissimilarity did show a deviation betwixt Chiliad and W (128.8 ms) (SEM 35.1, p = 0.006, event size = 1.36). In Figure 2 the marginal means are depicted in a dot and whiskers plot.

Table one. Results of Times S, 1000, and W expressed as mean, standard error of the mean past group and t-test results every bit p-values.

Effigy 2. Dot and whiskers plot of the estimated marginal means (EMM).
Equally a control analysis, inside the schizophrenia grouping, patients were asked subsequently the experiment if they "felt a difference" between Fourth dimension K and Time W. Four of the patients responded "yes" and 10 responded "no." For the subjects who felt a departure, their time W was −151 ms (SEM 51.6) and M was −51 ms (SEM 35.6). In dissimilarity the subjects who did not feel a difference, time M and W were 33.vi ms (SEM 27.8) and ten ms (SEM 25.6), respectively. In the subgroup analysis, there was a significant deviation between patients who felt a difference in W and those who did not (p = 0.0026).
Positive Symptoms
The mean total score for the SAPS was 19.1 (v.28 SEM). The presence of passivity symptoms was constitute in 6 patients, and the mean score was ix.67 (i.6 SEM). Five patients did not have passivity symptoms. The hateful total score for the hallucinations was 5.fifty (1.35 SEM), while the mean total score of hallucinations plus delusions was 16 (3.ix SEM). There was no correlation betwixt the subjective feeling of a difference between West and G trials and the severity of passivity symptoms. There were no correlations establish when taking into account the presence of hallucination symptoms solitary (mean 5.50, SEM 4.7) or with hallucinations symptoms together with the delusion symptoms (Tabular array two).
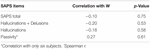
Tabular array 2. Correlation assay of SAPS with times Yard and W.
Motility Related Cortical Potentials
As a control analysis we analyzed the shape and the onsets of the MRCPs in both conditions (Westward and M). Due to the presence of artifacts, only 11 Salubrious volunteers and x patients were analyzed. Effigy 3 depicts the average over all subjects of the MRCPs for W trials and M trials. Table 3 summarizes the values (mean and SEM) of the amplitudes of the BPs and the onset of every MRCPs (i.e., BP1 and BP2). The amplitudes were computed at time 0, in correspondence with the EMG onset. 2 independent mixed models (for amplitude and onset, respectively) did not unveil any meaning difference for Group, Condition or the interaction (see the Supplementary File for the individual values). Thus, no postal service hoc tests were performed.

Table 3. Onset time of BP1 and BP2 for W and M in the patients and the healthy volunteers.
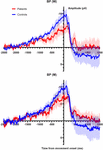
Effigy 3. (Tiptop) Averaged BP (main line) with standard mistake of the mean (shaded expanse) for all participants shown for electrode CZ for the W trials. Epoch begins ii.5 s prior to motion onset and one.5 southward after movement. (Bottom) Averaged BP (main line) with standard mistake of the mean (shaded area) for all participants shown for electrode CZ for the M trials. Epoch begins 2.5 s prior to motion onset and 1.5 s after move.
Word
We confirmed the findings of Libet et al. (1983) that in healthy volunteers, time W follows the onset of the BP just precedes time M. In contrast, patients with schizophrenia had a like onset of BP as good for you volunteers only in most at that place was no singled-out perception of W preceding M. Chiefly, the unconscious generation of voluntary motility was indistinguishable between the groups based on the BP assay, but the conscious perception of movement generation was clearly different between the groups. The feel of moving and willing most merged in the patient group. These results suggest that patients with schizophrenia do have an contradistinct timing of awareness of activity—or an dumb judgment of the sequence of events—compared to good for you individuals. This echoes results found in patients with functional (psychogenic) tremor and an contradistinct sense of agency where W was shifted later and Thousand and W were duplicate (Edwards et al., 2011). A similar consequence was found in subjects afflicted by dissociative seizures, brief episodes of disrupted awareness and behavioral control, who showed a very short difference between the reported timing of Chiliad and W (Jungilligens et al., 2019). Some other Libet-similar experiment, using the fMRI, plant that as well patients with mixed functional neurological disorder showed a reduced difference between the reported M and Westward (Baek et al., 2017).
Patients with schizophrenia have shown "hyperbinding" or "hyperassociation" in the conscious experience of events separated in time, meaning that patients over-associated their voluntary actions with a sensory cue (Haggard et al., 2003). In that experiment, patients "over-attributed" their bureau (Haggard et al., 2003). One interpretation of our results is that schizophrenic patients practise have a difference betwixt G and W only as healthy volunteers, merely due to excessive binding, those events are indistinguishable and reported similarly in time. This would advise that agency persists but that the process of willing a movement and moving are the same—not that the movement was nether alien command or other such phenomena. Alternatively, it is possible that they are non the same events but and then much closer in fourth dimension that a stardom is hard to detect with this methodology. This is consequent with other piece of work in patients with schizophrenia, which showed patients tend to underestimate a temporal interval between events (Franck et al., 2005).
After the job, patients were asked if they "felt a difference" between W and M, and four patients reported that they did. They performed similarly to salubrious volunteers with time Westward reported at 151 ms prior to motility onset. There was no relationship between their passivity scores or other disease measures to account for this difference in experience—and functioning on the task. Of note, all patients were on 2d-generation antipsychotic medications at the time of the task, which may have affected the passivity rating scales. Information technology is possible that the scales used were non adequately sensitive to notice this alter. In improver, the analysis was not powered to observe changes between private patient performance—simply to wait at patients and salubrious volunteers in a grouping fashion.
One of the important facets of Libet et al. (1983) original work was the presence of the Bereitschaftspotential preceding the study of fourth dimension W. He interpreted this phenomenon as a temporal progression of motion beginning with subconscious preparation for move followed past conscious awareness of the intention to move—quite proximal to the move itself. Other investigators take found BP1 amplitudes reduced and prolonged latency of BP1 in schizophrenic patients compared to salubrious volunteers (Dreher et al., 1999; Northoff et al., 2000). Nosotros did not observe significant differences in latency or in amplitude betwixt patients and healthy volunteers. In that location are certainly differences in study methodology that may account for the disparate results. In the previous studies, the patients were asked to make a self-paced motion of the finger every 4–5 s and fixate their gaze in the middle of a reckoner screen as opposed to our report where patients had their focus clearly on the clock and had to answer to questions of timing. This raises the question of "attending" versus "intention" in interpreting this piece of work.
Subjects in this experiment were asked later an event occurred to reconstruct the timing of their intention. Piece of work by Lau et al. (2004) showed that encephalon activity differed depending on whether the subject attended to the W task or the One thousand job. In the W task, in that location was increased blood period in dorsal prefrontal cortex, intraparietal sulcus and the pre-supplementary motor area (SMA). They suggested that the pre-SMA activation could be the reflection of intention; other piece of work suggests that the parietal cortex is important as well (Sirigu et al., 1999). In any case, the question of exactly what the subjects are attending to during the "intention" weather is an open up one (Eagleman, 2004). In all of these experiments including ours, subjects are asked to "access" this awareness of move—if one is not asked, does i nonetheless feel this sensation (Eagleman, 2004)? In a study by Matsuhashi and Hallett, they developed methodology to measure a time T, which was not dependent on subjective report of timing—only was thought to reflect the timing of the conscious intention to move (Matsuhashi and Hallett, 2008). They, as in our current work, bear witness that fourth dimension T was found after the encephalon had already begun unconscious preparations for motility (Matsuhashi and Hallett, 2008). This is once again consistent with a generalized preparation for motion that begins unconsciously and then progresses to a conscious awareness of intention. Whether this intention is generated prior to movement solely or modified later the motion due to a reconstruction of awareness, is non entirely clear.
An important indicate of view in the estimation of our results considers the relationship between W and the defect in the forward model of movement that the brain receives as the motor signal is generated (Frith et al., 2000). One model for motor control holds that movements are guided past mental representations made earlier the activity begins (Jordan, 1996). By this model, a motor command includes information on both the current limb position and desired limb position; the subtraction of these two states (the forwards model) requires the combination of known movements. Tracing a path that our mind has already made allows us to correct for errors quickly, integrate external sensory input into the movement plan, and learn from mental practice (Frith et al., 2000). Frith provided a model co-ordinate to which a single cognitive mechanism underlies the symptoms that characterize schizophrenia: an impairment in cocky-monitoring, or failure in meta-representation of one's own or others' internal states and beliefs. Such impairments might be involved in a disruption of the internal model and the efferent copy of motor programs, which would lead to a lack of awareness of the intended action. Such a disruption may likewise be behind the experience of delusions of control in which one's actions are experienced as if controlled by alien agents or forces. Thus, even if schizophrenics tin can make normal, coordinated movement, they demonstrate less of the benefits predicted by the forrad model: schizophrenics accept dumb cardinal error correction and more difficulty integrating unexpected interference during acts (Frith et al., 2000). Moreover, Maruff et al. (2003) found that the aberrant forward modeling in schizophrenics with passivity included impaired motor imagery: patients oftentimes failed to account for environmental restraints when making imagined voluntary movements.
More recently, some studies focused on ii distinct forward mechanisms: the integral forrad model, and the auxiliary forward model (Pickering and Clark, 2014). In the former, perception involves the employ of a forward model for the action. In the latter, the prediction mechanism is implemented past auxiliary circuits, like the CD, a re-create of the motor command used by the central nervous organisation to evaluate the sensory consequences of the actions, and it is this that seems altered in schizophrenia (Poletti et al., 2019). This approach has been used to explain the symptoms in schizophrenia: the generation of the forrard model is aberrant. Thus, the prediction of the sensory consequences of cocky-generated stimuli is inaccurate and, sometimes, fifty-fifty attributed to an external source (Wilkinson, 2015). We did non observe whatever relationship between the performance and either passivity or SAPS. The limited number of patients with passivity symptoms might partially account for this result but it may exist hypothesized that the abnormalities in the proposed forward model are more than related to the full general presence of positive symptoms, specific of the disorder, more than their severity.
In this current work, nosotros evidence that patients with schizophrenia differ from salubrious volunteers in their feel of the will to movement. This altered time sequence may generate an abnormal feel of causality and, even, an abnormal feel of conscious will itself – illusion or not (Wegner, 2003).
Data Availability Statement
The raw information supporting the conclusions of this article will be fabricated available by the authors, without undue reservation.
Ideals Argument
The studies involving human participants were reviewed and approved by National Found of Neurological Disorders and Stroke Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SP: concept and design, data analysis, data interpretation, and first version of manuscript. AT: information analysis and manuscript revision. MM: concept and data interpretation. VV: enrollments, data interpretation, and manuscript revision. EP: data recordings and manuscript revision. FN and ZM: data recordings, information analysis, and manuscript revision. MH: concept and blueprint, information interpretation, and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This inquiry was supported past the Intramural Research Program of the NIH, NINDS, where the clinical research was carried out. AT was supported past a joint grant from the John Templeton Foundation and the Fetzer Establish. The opinions expressed in this publication are those of the writer(south) and do not necessarily reflect the views of the John Templeton Foundation or the Fetzer Institute.
Conflict of Involvement
The authors declare that the inquiry was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential conflict of interest.
The treatment editor declared a past co-authorship with one of the authors EP.
Acknowledgments
The authors thank Dr. Sarah Kranick and Dr. José A. Apud for their help and aid in the first steps of this study.
Supplementary Material
The Supplementary Material for this article can exist found online at: https://www.frontiersin.org/articles/10.3389/fnins.2020.574472/full#supplementary-fabric
References
American Psychiatric and Association (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th Edn. Washington, DC: APA.
Google Scholar
Andreasen, Due north. C. (1984). The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: U.O.I. Press.
Google Scholar
Baek, K., Doñamayor, Due north., Morris, L. S., Strelchuk, D., Mitchell, Southward., Mikheenko, Y., et al. (2017). Impaired sensation of motor intention in functional neurological disorder: implications for voluntary and functional motility. Psychol. Med. 47, 1624–1636. doi: 10.1017/s0033291717000071
PubMed Abstract | CrossRef Full Text | Google Scholar
Dreher, J. C., Trapp, W., Banquet, J. P., Keil, M., Gunther, Due west., and Burnod, Y. (1999). Planning dysfunction in schizophrenia: harm of potentials preceding fixed/free and unmarried/sequence of self-initiated finger movements. Exp. Brain Res. 124, 200–214. doi: 10.1007/s002210050615
PubMed Abstract | CrossRef Full Text | Google Scholar
Edwards, K. J., Moretto, G., Schwingenschuh, P., Katschnig, P., Bhatia, K. P., and Haggard, P. (2011). Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia 49, 2791–2793. doi: x.1016/j.neuropsychologia.2011.05.021
PubMed Abstract | CrossRef Full Text | Google Scholar
Offset, M., Spitzer, R., Gibbon, Grand., and Williams, J. (2002). Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute.
Google Scholar
Franck, N., Posada, A., Pichon, S., and Haggard, P. (2005). Altered subjective time of events in schizophrenia. J. Nerv. Ment. Dis. 193, 350–353. doi: 10.1097/01.nmd.0000161699.76032.09
CrossRef Full Text | Google Scholar
Frith, C. D., Blakemore, S.-J., and Wolpert, D. M. (2000). Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res. Rev. 31, 357–363. doi: ten.1016/s0165-0173(99)00052-ane
CrossRef Full Text | Google Scholar
Gordon, M., McClure, F. D., and Aylward, 1000. P. (2002). The Gordon Diagnostic Organisation Interpretive Guide, 3rd Edn. DeWitt, NY: GSI Publications.
Google Scholar
Gordon, M., and Mettelman, B. (1987). Technical Guide to the Gordon Diagnostic Arrangement (GDS). DeWitt, NY: Gordon Systems.
Google Scholar
Graham, K. T., Martin-Iverson, M. T., Holmes, N. P., Jablensky, A., and Waters, F. (2014). Deficits in bureau in schizophrenia, and additional deficits in trunk image, torso schema, and internal timing, in passivity symptoms. Front. Psychiatry 5:126. doi: x.3389/fpsyt.2014.00126
PubMed Abstruse | CrossRef Full Text | Google Scholar
Graham-Schmidt, K. T., Martin-Iverson, M. T., and Waters, F. A. V. (2018). Self- and other-agency in people with passivity (first rank) symptoms in schizophrenia. Schizophr. Res. 192, 75–81. doi: 10.1016/j.schres.2017.04.024
PubMed Abstruse | CrossRef Full Text | Google Scholar
Haggard, P., Martin, F., Taylor-Clarke, M., Jeannerod, M., and Franck, Northward. (2003). Awareness of activeness in schizophrenia. Neuroreport 14, 1081–1085. doi: x.1097/01.wnr.0000073684.00308.c0
CrossRef Total Text | Google Scholar
Herbener, E. S., and Harrow, One thousand. (2019). Course and symptom and functional correlates of passivity symptoms in schizophrenia: an 18-yr multi-follow-upwards longitudinal study. Psychol. Med. 16, 1–viii. doi: 10.1017/s0033291719003428
PubMed Abstruse | CrossRef Full Text | Google Scholar
Jordan, M. I. (1996). "Computational aspects of motor command and motor learning," in Handbook of Perception and Action: Motor Skills, eds E. H. Heuer and Eastward. Due south. Keele (New York, NY: Academic Press). doi: x.4324/9781315185613-1
CrossRef Full Text | Google Scholar
Jungilligens, J., Wellmer, J., Schlegel, U., Kessler, H., Axmacher, N., and Popkirov, S. (2019). Impaired emotional and behavioural awareness and control in patients with dissociative seizures. Psychol. Med. eighteen, i–9. doi: 10.1017/s0033291719002861
PubMed Abstract | CrossRef Full Text | Google Scholar
Karp, B. I., Parter, S., Toro, C., and Hallett, M. (1996). Simple motor tics may be preceded past a premotor potential. J. Neurol Neurosurg. Psychiatry 61, 103–106. doi: 10.1136/jnnp.61.1.103
PubMed Abstruse | CrossRef Full Text | Google Scholar
Libet, B., Gleason, C. A., Wright, E. W., and Pearl, D. G. (1983). Time of witting intention to deed in relation to onset of cognitive activity (readiness-potential): the unconscious initiation of a freely voluntary human action. Brain 106, 623–642. doi: 10.1093/brain/106.3.623
PubMed Abstract | CrossRef Full Text | Google Scholar
Maruff, P., Wilson, P., and Currie, J. (2003). Abnormalities of motor imagery associated with somatic passivity phenomena in schizophrenia. Schizophr. Res. 60, 229–238. doi: 10.1016/s0920-9964(02)00214-1
CrossRef Full Text | Google Scholar
Motulsky, H. J., and Brown, R. Due east. (2006). Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7:123. doi: 10.1186/1471-2105-7-123
PubMed Abstract | CrossRef Total Text | Google Scholar
Northoff, 1000., Pfennig, A., Krug, M., Danos, P., Leschinger, A., Schwarz, A., et al. (2000). Delayed onset of late move-related cortical potentials and abnormal response to lorazepam in catatonia. Schizophr. Res. 44, 193–211. doi: 10.1016/s0920-9964(99)00189-9
CrossRef Total Text | Google Scholar
Poletti, M., Tortorella, A., and Raballo, A. (2019). Dumb corollary discharge in psychosis and at-gamble states: integrating neurodevelopmental, phenomenological, and clinical perspectives. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 832–841. doi: 10.1016/j.bpsc.2019.05.008
PubMed Abstract | CrossRef Full Text | Google Scholar
R Core Team (2018). R: A Linguistic communication and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Google Scholar
Schnell, K., Heekeren, K., Daumann, J., Schnell, T., Schnitker, R., Moller-Hartmann, Due west., et al. (2008). Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain 131, 2783–2797. doi: x.1093/encephalon/awn184
PubMed Abstract | CrossRef Full Text | Google Scholar
Sirigu, A., Daprati, E., Pradat-Diehl, P., Franck, Northward., and Jeannerod, One thousand. (1999). Perception of cocky-generated movement post-obit left parietal lesion. Brain 122, 1867–1874. doi: 10.1093/brain/122.10.1867
PubMed Abstract | CrossRef Full Text | Google Scholar
Tombaugh, T. N. (2004). Trail making test A and B: normative data stratified by historic period and didactics. Arch. Clin. Neuropsychol. 19, 203–214. doi: 10.1016/s0887-6177(03)00039-8
CrossRef Full Text | Google Scholar
Wechsler, D. (1981). Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation.
Google Scholar
Wechsler, D. (1987). Wechsler Memory Scale-Revised Transmission. New York NY: The Psychological Corporation.
Google Scholar
Wegner, D. G. (2003). The heed's best fox: how nosotros feel witting will. Trends Cogn. Sci. seven, 65–69. doi: 10.1016/s1364-6613(03)00002-0
CrossRef Full Text | Google Scholar
Yao, B., Neggers, S. F. Westward., Rolfs, M., Rosler, L., Thompson, I. A., Hopman, H. J., et al. (2019). Structural thalamofrontal hypoconnectivity is related to oculomotor corollary discharge dysfunction in schizophrenia. J. Neurosci. 39, 2102–2113. doi: ten.1523/jneurosci.1473-18.2019
PubMed Abstract | CrossRef Full Text | Google Scholar
0 Response to "To Be Seen as Generated and Fashioned by Volition"
Post a Comment